[新しいコレクション] homogeneous and heterogeneous reactions examples 596158-Homogeneous and heterogeneous reactions examples
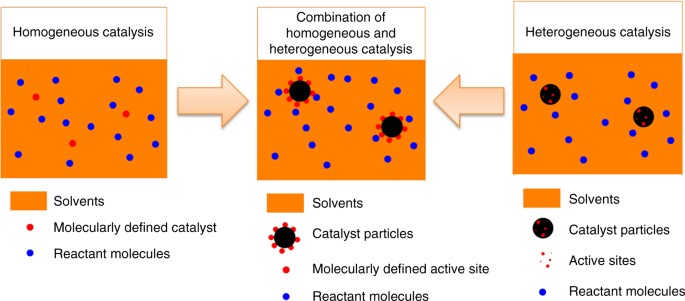
Examples of homogeneous reactions are the combination of natural gas and oxygen to produce flame or a reaction of aqueous solutions of acid and bases Out of the two homogeneous and heterogeneous reactions, the former is easy to understand as the nature of homogeneous reactions solely depends upon the nature of the interaction of the reactantsHomogeneous and Heterogeneous Reaction Video Lecture from Chemical Equilibrium Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEETAndroid ApplicatioHomogeneous Catalyst Homogeneous catalysts have advantages over heterogeneous catalysts such as possibility of carrying out the reaction at milder conditions, higher activity, and selectivity, ease of spectroscopic monitoring, and controlled and tunable reaction sites From Handbook of Solvents (Second Edition), Volume 2, 14 Related terms

Differences Between Homogeneous And Heterogeneous Ef Processes Download Table
Homogeneous and heterogeneous reactions examples
Homogeneous and heterogeneous reactions examples-The whole world is a solid heterogeneous mixture! A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to learn more interesting topics in Chemistry
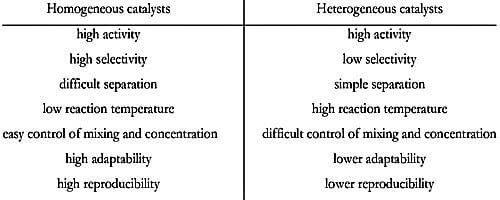



Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study
A heterogeneous equilibrium reaction, in contrast, is a reaction where the products and reactants are in different phases For example, one compound or molecule mightHomogeneous catalysis takes place when the catalyst and the other reactants are all dissolved in the same solution Heterogeneous catalysis typically involves the use of a catalyst that is insoluble, or perhaps only weakly soluble, in the solution in which the reaction takes placeExamples include sand and sugar, salt and gravel, a basket of produce, and a toy box filled with toys Mixtures in two or more phases are heterogeneous mixtures Examples include ice
A catalyst is a compound used to help a reaction occur faster by lowering the activation energy There are two types of catalysts, homogeneous and heterogeneous A homogeneous catalyst is aA complete answer to the question What are Examples of homogeneous mixtures and heterogeneous mixtures? For example, the physical eye can pick up the substances that make up this type of mixture because they are large enough to be seen Like homogeneous mixtures, examples of heterogeneous mixtures can include solids, liquids, and gases Some liquid examples include salad dressing and red wine vinegar A gas example can include air with clouds in
About heterogeneous mixture homogeneous mixture worksheet The heterogeneous mixture – homogeneous mixture worksheet with answer key is below The worksheet gives common examples of mixtures, in addition to some pure, unmixed substances Learn how to classify these examples of mixtures belowHomogeneous reaction, any of a class of chemical reactions that occur in a single phase (gaseous, liquid, or solid), one of two broad classes of reactions—homogeneous and heterogeneous—based on the physical state of the substances present The most important of homogeneous reactions are the reactions between gases (eg, the combination of common Examples of homogeneous mixtures include Salty water — a mixture of salt and water Ruby — a mixture of Al 2 O 3 and Cr 2 O 3 Gasoline — a mixture of various hydrocarbons Brass — a mixture of Cu and Zn Air without clouds — a mixture of various gases Heterogeneous mixtures contain two or more components that can be seen, which can
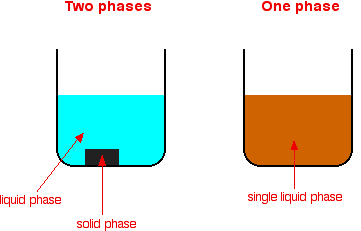



Types Of Catalysis
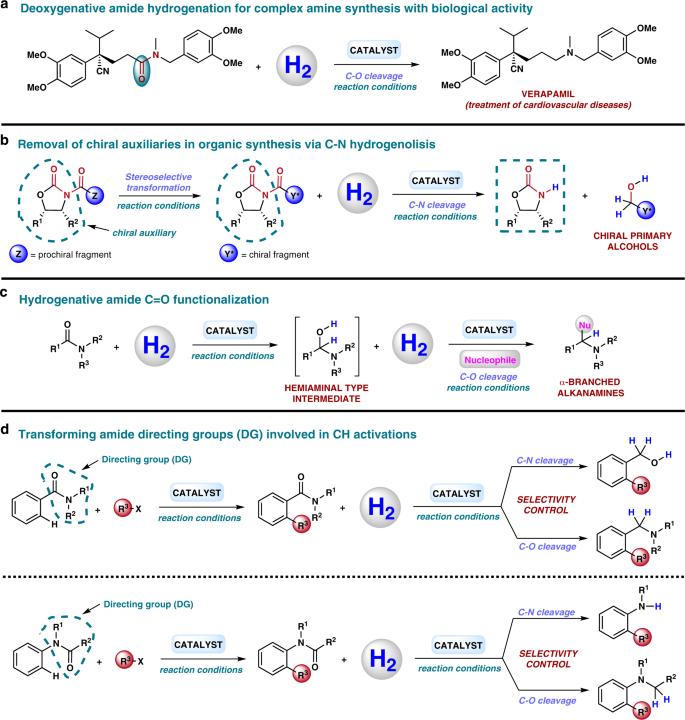



Homogeneous And Heterogeneous Catalytic Reduction Of Amides And Related Compounds Using Molecular Hydrogen Nature Communications
Acid catalysis, organometallic catalysis, and enzymatic catalysis are examples of homogeneous catalysis Homogeneous catalysts are used in variety of industrial applications, as they allow for an increase in reaction rate without an increase in temperature Click to see full answer Likewise, people ask, what is heterogeneous catalysis give examples?We notice that the solid calcium hydroxide is in equilibrium with its saturated solution Writing the equilibrium constant for heterogeneous reactions is different from that of the homogeneous reactions For example, consider the thermal dissociation of calcium carbonate into calcium oxide and carbon dioxideHomogeneous vs heterogeneous catalysis Dr habil Marko Hapke 3 3 Heterogeneous Catalysis Homogeneous Catalysis Catalyst and reactant(s) are in the same phase Catalyst and reactant(s) are in different phases Definitions General features Different reaction phases possible „classic"



Mauvia Cre Ppt
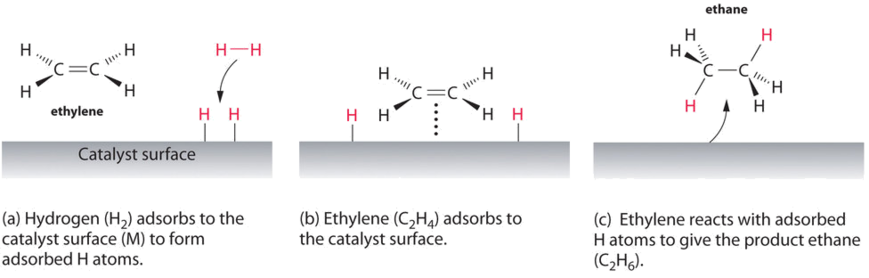



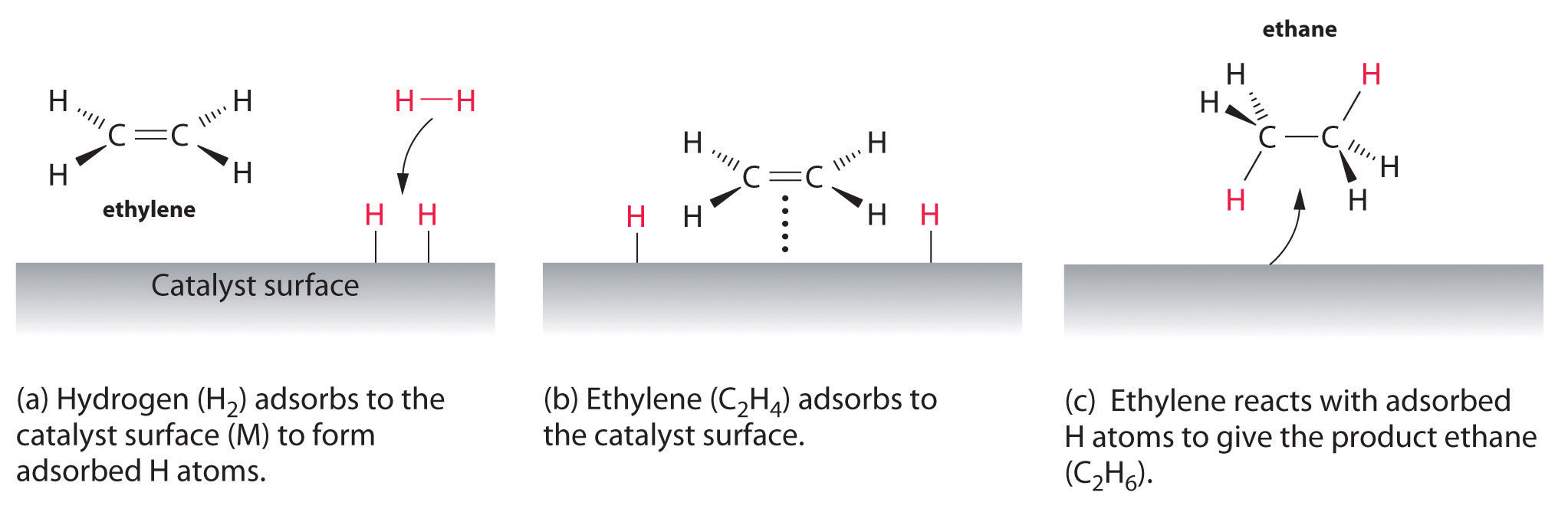
14 7 Catalysis Chemistry Libretexts
An example of a homogeneous catalysis is one wherein the catalyst and the reactants are in the gaseous phase An example is acid catalysis The acid dissolved in water produces a proton that speeds up chemical reaction, such as in the hydrolysis of estersAn example of a colloid is milk Milk is a mixture of liquid butterfat globules dispersed and suspended in water Colloids are generally considered heterogeneous mixtures, but have some qualities of homogeneous mixtures as well Interesting Facts about Mixtures Smoke is a mixture of particles that are suspended in the airUNESCO – EOLSS SAMPLE CHAPTERS INORGANIC AND BIOINORGANIC CHEMISTRY – Vol II Homogeneous and Heterogeneous Catalysis Erica Farnetti, Roberta Di Monte and Jan Kašpar ©Encyclopedia of Life Support Systems (EOLSS) terms ab,, and cd,, represent the stoichiometric coefficients of the reactionFor such a reaction we can define the reaction rate as




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms




15 4 Heterogeneous Reactions Youtube
What are 10 examples of heterogeneous mixtures?The esterification reaction is another wellknown example of homogeneous catalysis 7,8 In this reaction, sulfuric acid dissolves in ethanol/ethanoic acid Although in this catalysis there is a good contact with reactants, separation of the catalysis after the If you look closely at sand from a beach, you can see the different components, including shells, coral, sand, and organic matter It's a heterogeneous mixture If, however, you view a large volume of sand from a distance, it's impossible to discern the different types of particles The mixture is homogeneous




Difference Between Homogeneous Mixture And Heterogeneous Mixture



Nitrogen Reduction
For example, adding dye to water will create a heterogeneous solution at first, but will become homogeneous over time Entropy allows for heterogeneous substances to become homogeneous over time A heterogeneous mixture is a mixture of two or more compoundsHello friend heterogenous is derived from greek word heteromeans different, genouskind In chemistry,heterogeneous mixture is a mixture which is formed from mixture of 2 different phased substances ie liquid and gas ,solid & liquid etc A reactTypical examples involve a solidcatalyst with the reactants as either liquids or gases Note It is important that you remember the difference between the two terms heterogeneousand homogeneous heteroimplies different(as in heterosexual) Heterogeneous catalysis has the catalyst in a different phase from the reactants




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications
A mixture consists of sugar in water is a homogeneous mixture because we can't see particles of sugar in the water, as they are dissolved thoroughly A mixture consisting of oil in water is an example of the heterogeneous mixture as the oil cannot be mixed in the water and we can easily see them I hope you like my post about "Difference In heterogeneous catalysis, the reaction starts at the surface of the solid catalyst and so it is also known as surface catalysis Example Manufacture of H 2 SO 4 by contact process involves oxidation of SO 2 into SO 3 in presence of V 2 O 5 (solid) as catalyst A homogeneous equilibrium is one in which all species are present in the same phase Common examples include gasphase or solution reactions A heterogeneous equilibrium is one in which species exist in more than one phase Common examples include reactions involving solids and gases, or solids and liquids



Catalysis



Exe
Some examples of the homogeneous mixtures are known as sugar, salt, water, dye, air, and blood A heterogeneous mixture has a clear identifying property where one can see various different components of the mixture It is a reaction system where the products and the reactants are found in two or more phasesHomogeneousheterogeneous reactions in peristaltic flows of nonNewtonian fluids Even such study is yet not presented for the viscous fluid However such consideration is quite important because many chemically reacting systems involve both homogeneous and heterogeneous reactions, with examples occurringTypically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixture Catalysis Note the lowered activation energy of the catalyzed pathway Examples of Homogeneous Catalysts Acid catalysis, organometallic catalysis, and enzymatic catalysis are examples of homogeneous catalysis




Homogeneous Catalysis Wikipedia




Explain The Difference Between A Homogeneous And Heterogeneous Catalyst Give An Example Of Each Youtube
The key difference between homogeneous and heterogeneous equilibrium is that in homogeneous equilibrium, the reactants and products are in the same phase of matter whereas, in heterogeneous equilibrium, the reactants and products are in different phases Equilibrium is a state in which the concentrations of reactants and products remain constant There are two Examples of homogeneous mixtures include air, saline solution, most alloys, and bitumen Examples of heterogeneous mixtures include sand, oil and water, and chicken noodle soup What is solid solid heterogeneous mixture?Catalysts are generally divided into two types, those that are in the same phase as the reactants (homogeneous catalysts) and those that belong to a different phase (heterogeneous catalysts) The first of the two reactions in this demonstration is an example of a homogeneous catalyst




Ppt On Reaction Kinetics Powerpoint Slides




Chemical Kinetics Prezentaciya Onlajn
In contrast, a system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium, such as the reaction of a gas with a solid or liquid As noted in the previous section, the equilibrium constant expression is actually a ratio of activitiesA homogeneous equilibrium is one in which all species are present in the same phase Common examples include gasphase or solution reactions A heterogeneous equilibrium is one in which species exist in more than one phase Common examples include reactions involving solids and gases, or solids and liquidsThese are known as heterogeneous catalytic reactions Eg It applies to reactions in the gas phase and even in solids Which one of following reaction is an example of homogeneous and heterogeneous catalysis 1 See answer frinkysharvlo is waiting for your help An example of this process is shown below



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science
Heterogeneous reaction, any of a class of chemical reactions in which the reactants are components of two or more phases (solid and gas, solid and liquid, two immiscible liquids) or in which one or more reactants undergo chemical change at an interface, eg, on the surface of a solid catalyst The reaction of metals with acids, the electrochemical changes that occur in batteries and electrolytic cells, and the phenomena of corrosion are part of the subject of heterogeneous reactionsSome examples of homogeneous reactions are oxyacetylene torch burning, carbon monoxide reacting with oxygen in air, HCl reacting with NaOH in water, etc Some examples of heterogeneous reactions are coal burning in air, iron rusting under water, sodium metal reacting with water, etc I hope that this helps clear up the differenceIn heterogeneous equilibrium, substances are in different phases Key Terms equilibrium The state of a reaction in which the rates of the forward and reverse reactions are the same heterogeneous solution A solution composed of different states of matter homogeneous solution A solution composed of matter that all exists in the same state



Chapter 17 Reaction Rates Allyson S Site
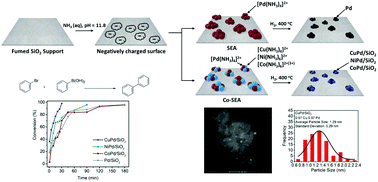



Heterogeneous Catalysis By Ultra Small Bimetallic Nanoparticles Surpassing Homogeneous Catalysis For Carbon Carbon Bond Forming Reactions Nanoscale Rsc Publishing
The key difference between homogeneous and heterogeneous reactions is that the reactants and products that take part in homogeneous reactions are in the same phase whereas the reactants and products in heterogeneous reactions are in different phases The homogeneity and heterogeneity are two chemical concepts that we describe regarding theExamples of homogeneous mixture A glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample You can't easily separate the lemon juice from the water;• Apple juice is homogeneous • Orange juice with pulp is heterogeneous • Chocolate dough is homogeneous • Italian salad dressing is heterogeneous



Homogeneous Reactions




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms
Homogeneous Reactions In homogeneous reactions the reaction mixture contains one single phase (gas, liquid or solid) When materials react to form products, there may be a single reaction or multiple reactions occurring There are ways to determine whether a single stoichiometric equation and single rate equation ( single reaction ), orDispersed phase and continuous phase The insoluble particles of a colloid do not settle down completely as the particles are small in size, usually ranging between 107 to 103 cm Examples of a heterogeneous mixture Colloid A colloid is an example of a heterogeneous mixture where the components exist in two distinct phases;




A Sustainable Procedure Combining The Advantages Of Both Homogeneous And Heterogeneous Catalysis For The Heck Matsuda Reaction




Write Some Simple Examples Of Homogeneous And Heterogeneous Equilibrium Brainly In
Homogeneous reactions are chemical reactions in which the reactants are in the same phase, while heterogeneous reactions have reactants in two or more phases Reactions that take place on the surface of a catalyst of a different phase are also heterogeneous A reaction between two gases, two liquids or two solids is homogeneousFor example, a salad Unlike homogeneous mixtures, in heterogeneous mixtures it is very easy to identify, even with the naked eye, what are the different components that make them up This makes it much easier to separate these mixes at the same



Homogeneous Vs Heterogeneous Catalysts Basic Introduction Youtube




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com



Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




Mauvia Cre Ppt




Difference Between Homogenous And Heterogenous Catalysis With Example Brainly In




Homogeneous And Heterogeneous Reaction Chemical Equilibrium Chemistry Class 11 Youtube




Catalysis Boundless Chemistry
:max_bytes(150000):strip_icc()/catalystenergydiagram-56a12b265f9b58b7d0bcb2fe.jpg)



Heterogeneous Reaction Definition




1 Schematic Diagram Of The Reaction Pathes In Homogeneous Download Scientific Diagram
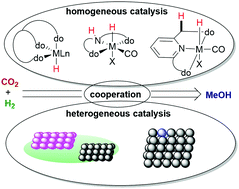



Homogeneous And Heterogeneous Catalysts For Hydrogenation Of Co2 To Methanol Under Mild Conditions Chemical Society Reviews Rsc Publishing




Ethiopia Learning Chemistry Grade 11 Page 217 In English




Factors Affecting Homogeneous Reactions Concept Chemistry Video By Brightstorm




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms




Question 02 A Being A Process Engineer Give Chegg Com
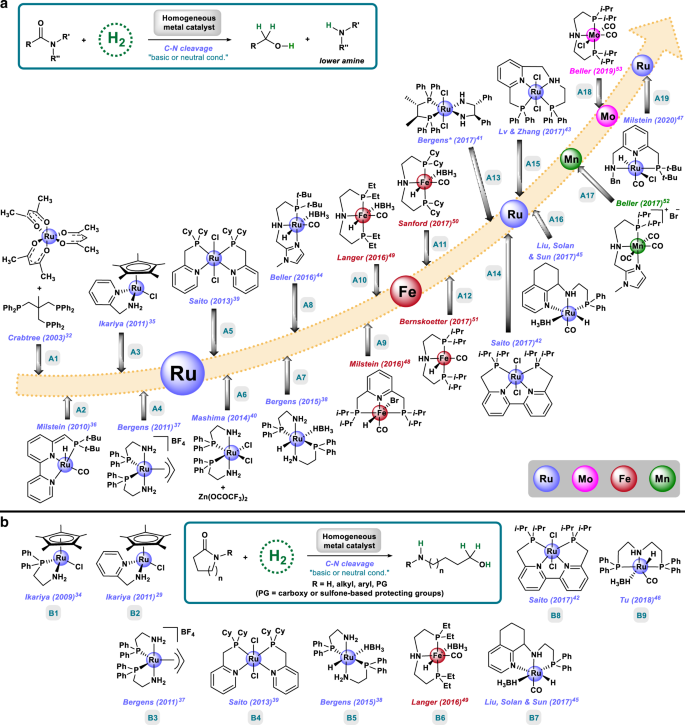



Homogeneous And Heterogeneous Catalytic Reduction Of Amides And Related Compounds Using Molecular Hydrogen Nature Communications




Crossing The Divide Between Homogeneous And Heterogeneous Catalysis In Water Oxidation Pnas
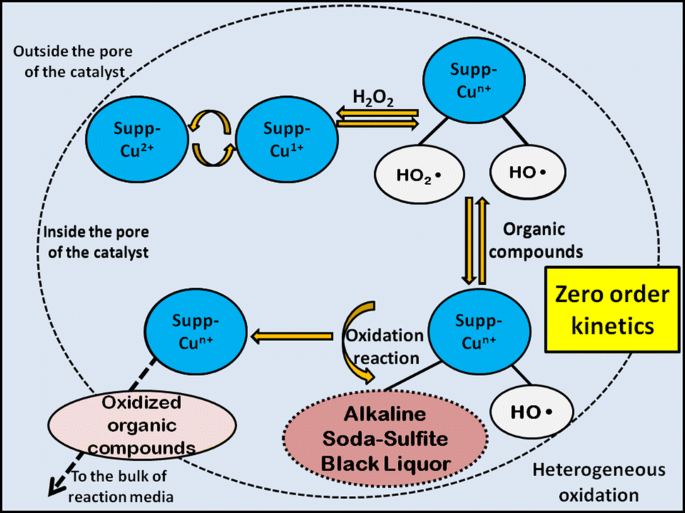



Kinetic Modeling Of A Heterogeneous Fenton Type Oxidative Treatment Of Complex Industrial Effluent Springerlink




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study




Solid Phase Catalysis In Continuous Flow Syrris Chemistry Blog




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table




Advantages And Disadvantages Of Homogeneous And Heterogeneous Catalysts Download Scientific Diagram




Rate Laws Lec 2 Week 2 Recall The Rate Of Reaction The Rate Of Formation The Rate Of Disappearing Units For The Rate Of Reaction Stoichiometrec Ppt Download




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com




Give Four Examples Of Heterogeneous Catalysis




Give Four Examples Of Heterogeneous Catalytic Reactions




Heterogeneous Catalysis Wikipedia




10 Examples Of Mixtures



Mauvia Cre Ppt



1




Heterogeneous Catalysis Wikipedia




Homogeneous And Heterogeneous Mechanochemical Reactions
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures
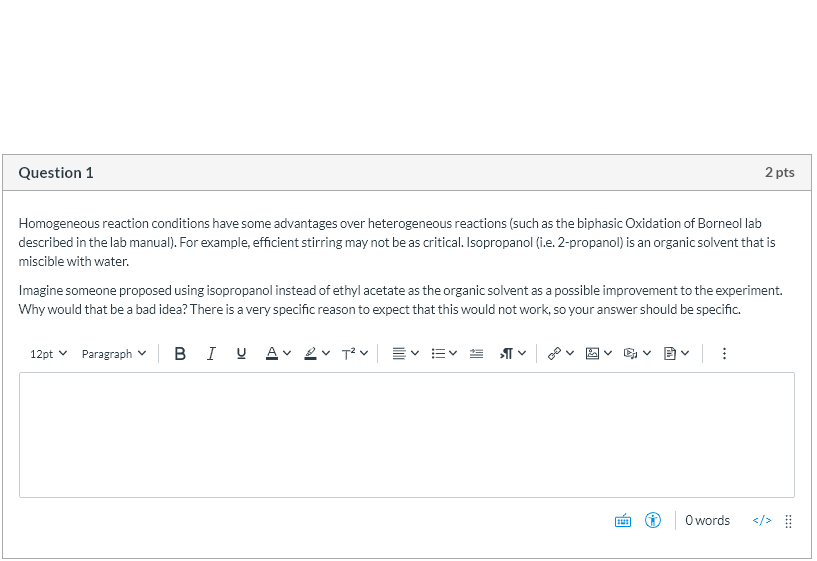



Question 1 2 Pts Homogeneous Reaction Conditions Have Chegg Com




Question 8 10 Points A What Is The Difference Chegg Com




Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World
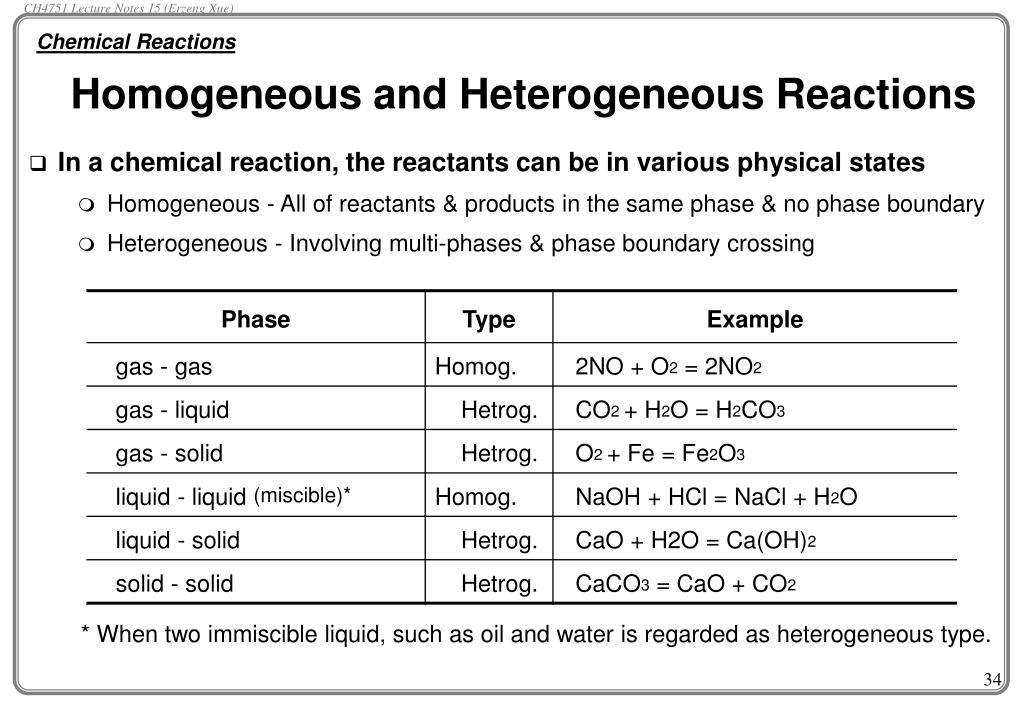



Ppt Introductory Chemistry B Ch4751 Lecture Notes 11 Dr Erzeng Xue Powerpoint Presentation Id




Difference Between Homogeneous Catalysis And Heterogeneous Catalysis Surface Chemistry Youtube




For Homogeneous Equilibrium Why Are Liquids And Solids Included In The Equilibrium Constant When They Aren T In Heterogeneous Equilibria Chemistry Stack Exchange
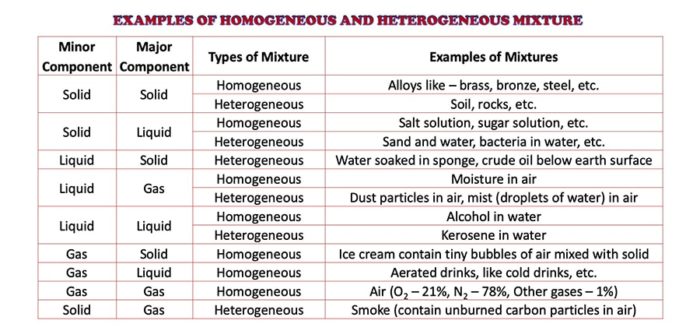



Homogeneous Heterogeneous Mixture Definition Examples Selftution
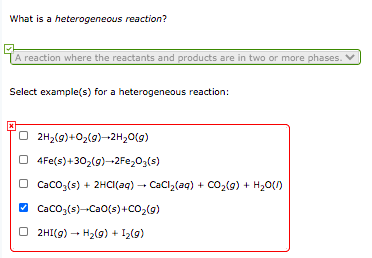



What Is A Heterogeneous Reaction




Homogeneous And Heterogeneous Reactions For Gasification Download Table



Homogeneous Heterogeneous Reactions In Peristaltic Flow With Convective Conditions



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora




Q1 Define Homogenous And Heterogeneous Mixture And Chegg Com



1




The Position Of Equilibrium Topic 7 2 Equilibrium




What Are Some Examples Of Homogeneous Reactions Quora




Escaping Undesired Gas Phase Chemistry Microwave Driven Selectivity Enhancement In Heterogeneous Catalytic Reactors Science Advances




3 Examples Of Heterogeneous And Homogeneous Boundary Layer Reactions Download Table
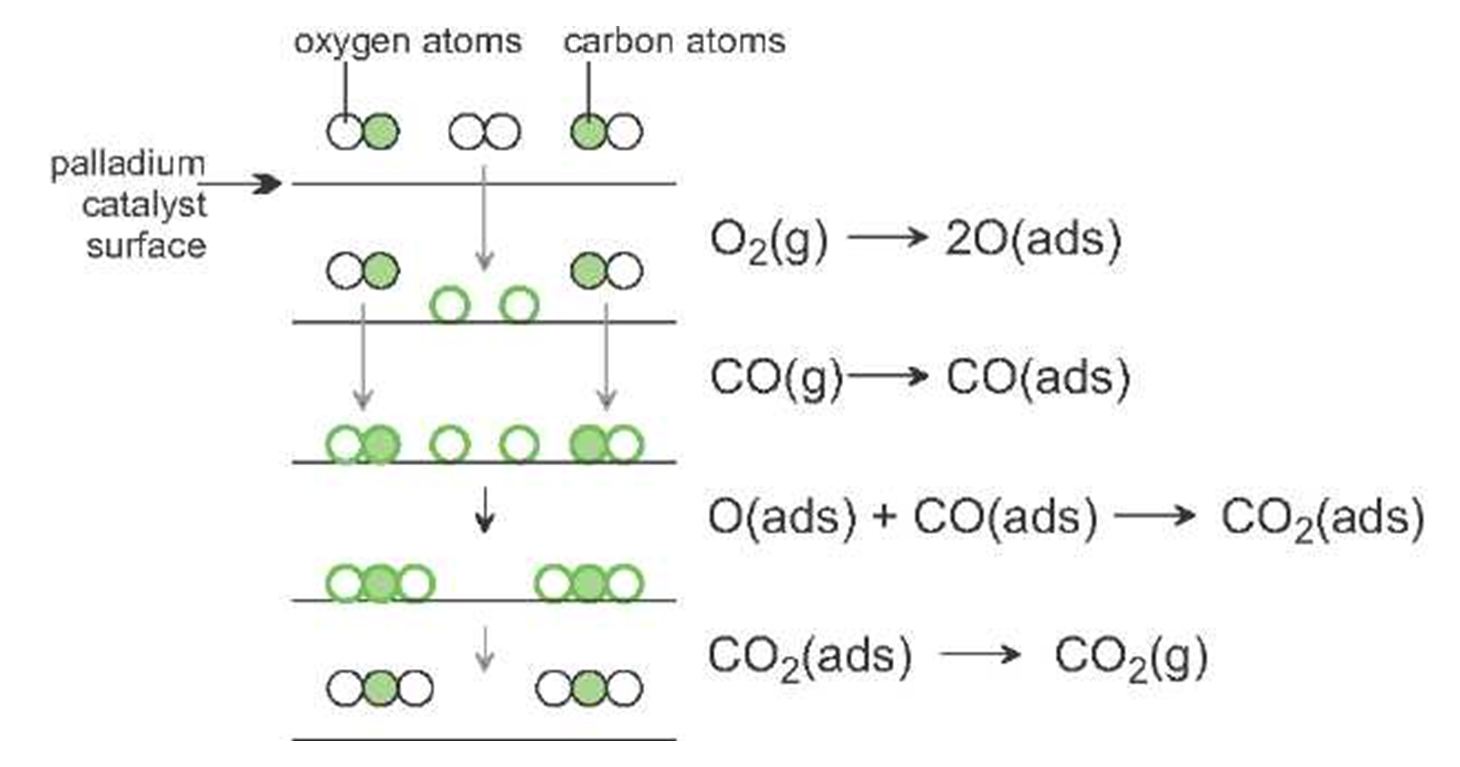



Catalysis In Industry




Catalysis Fundamentals Chemical Engineering Page 1
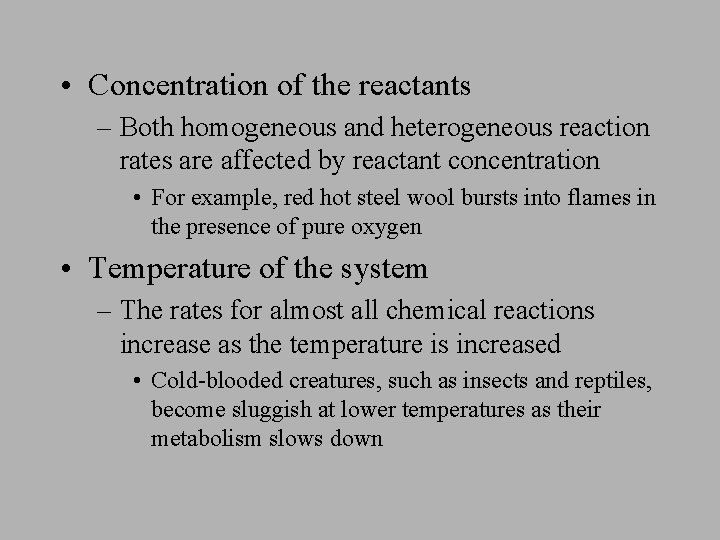



Chapter 15 Kinetics The Speed With Which The




Doc Catalysis Idris Seun Academia Edu




Is Spaghetti Sauce Homogeneous Or Heterogeneous
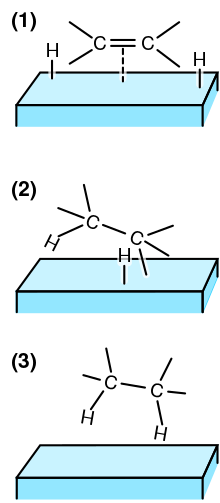



Heterogeneous Catalysis Wikipedia




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com




Heterogeneous Catalysis Wikipedia




Evolution Of Isolated Atoms And Clusters In Catalysis Trends In Chemistry




Review Notes 1




Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Exam Heterogeneous Mixture Chemistry Basics Biology Facts




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms
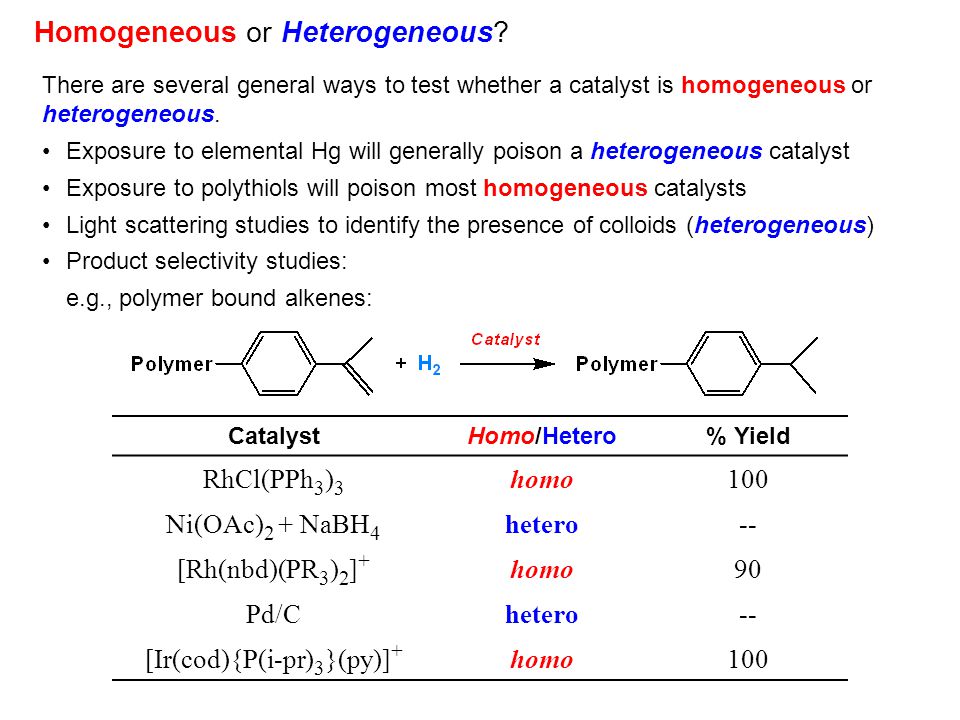



Homogeneous Catalysis Introduction Ppt Video Online Download




What Is An Example Of Heterogeneous Reaction
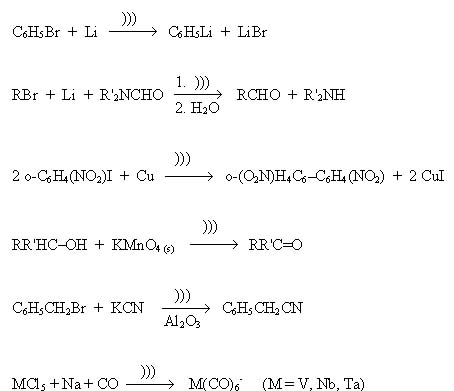



Sonochemistry The Suslick Research Group




Catalysis Of Chemical Reactions Homogeneous Heterogeneous Catalysis



Types Of Catalysis




Homogeneous Catalysis Catalysis Heterogeneous Catalysis
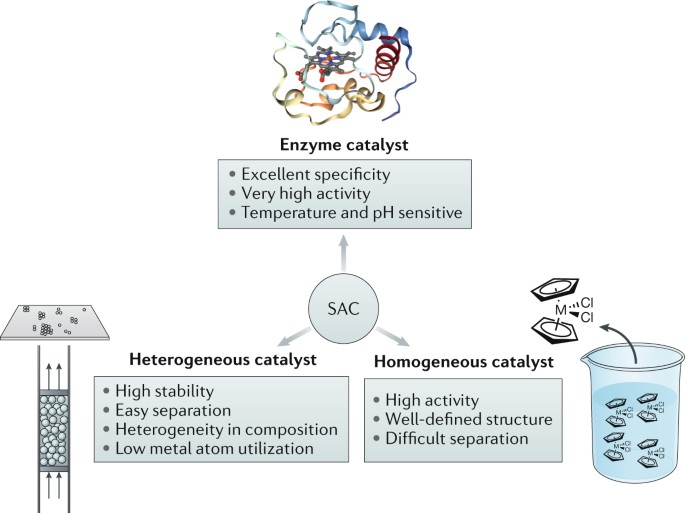



Heterogeneous Single Atom Catalysis Nature Reviews Chemistry




26 Homogeneous And Heterogeneous Catalysis Chapter
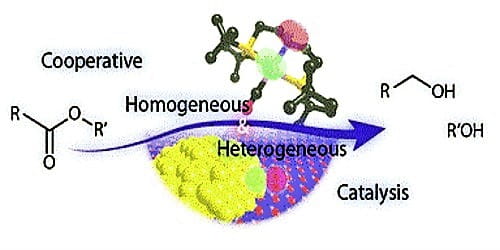



Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study




Comparative Investigation Of Homogeneous And Heterogeneous Bronsted Base Catalysts For The Isomerization Of Glucose To Fructose In Aqueous Media Sciencedirect
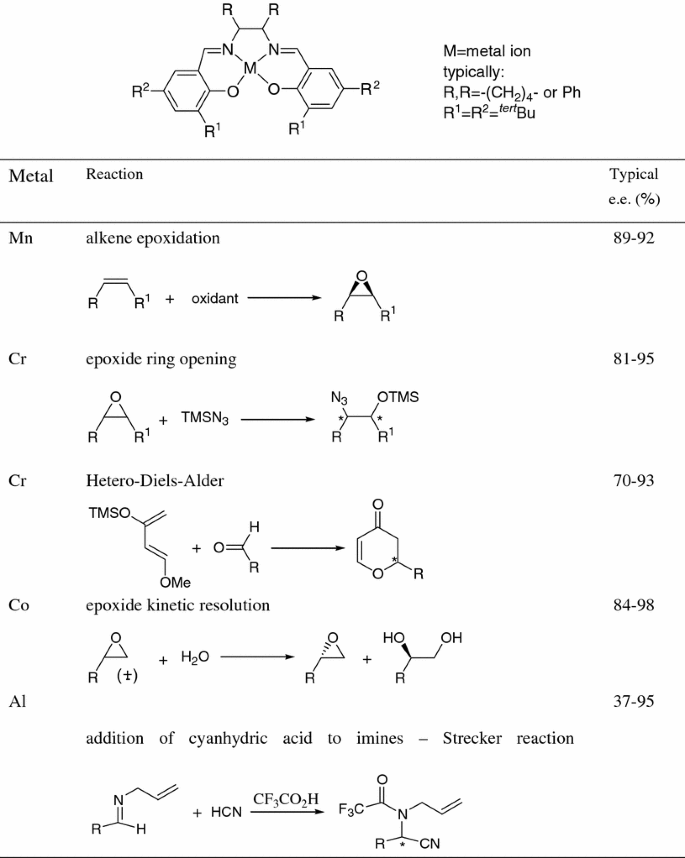



Crossing The Borders Between Homogeneous And Heterogeneous Catalysis Developing Recoverable And Reusable Catalytic Systems Springerlink



Www Ethz Ch Content Dam Ethz Special Interest Chab Icb Van Bokhoven Group Dam Coursework Catalysis 17 Homogeneous Heterogeoenous Catalysis Mesoporousmaterials Pdf



1



2




A Comparative Study Of Casson Fluid With Homogeneous Heterogeneous Reactions Sciencedirect



23 5 Features Of Homogeneous Catalysis A Catalyst
コメント
コメントを投稿